Chemistry 2e
The comprehensive contents from this book, combined with Odigia’s Teaching and Learning Tools have everything you need to engage, collaborate, track and assess your students.
This course includes:
276
practice questions
105
engagement activities

598
assessment questions
Helping Teachers Do What They Do Best: Teach

Customize
Use our courses as is or easily customize them to fit your teaching style and the needs of your students. You can add your favorite resources, hide and show our existing content and pre-built assessments, or make them your own. Everything your students need, in one place!

Engage and Collaborate
Odigia combines learning materials, discussions, and tools to create a familiar social experience for students allowing you to easily connect and redirect students attention.

Track
See how much time students are spending on different areas of the course, which areas are creating the most amount of engagement and identify topics the students are struggling with. Flag and provide feedback on assignments to proactively meet individual students' needs.

Assess
Game theory allows students to monitor their progress visually and motivates them to stay on track. Students can see exactly what activities they need to complete, which ones have been flagged and compare their progress against the overall class.
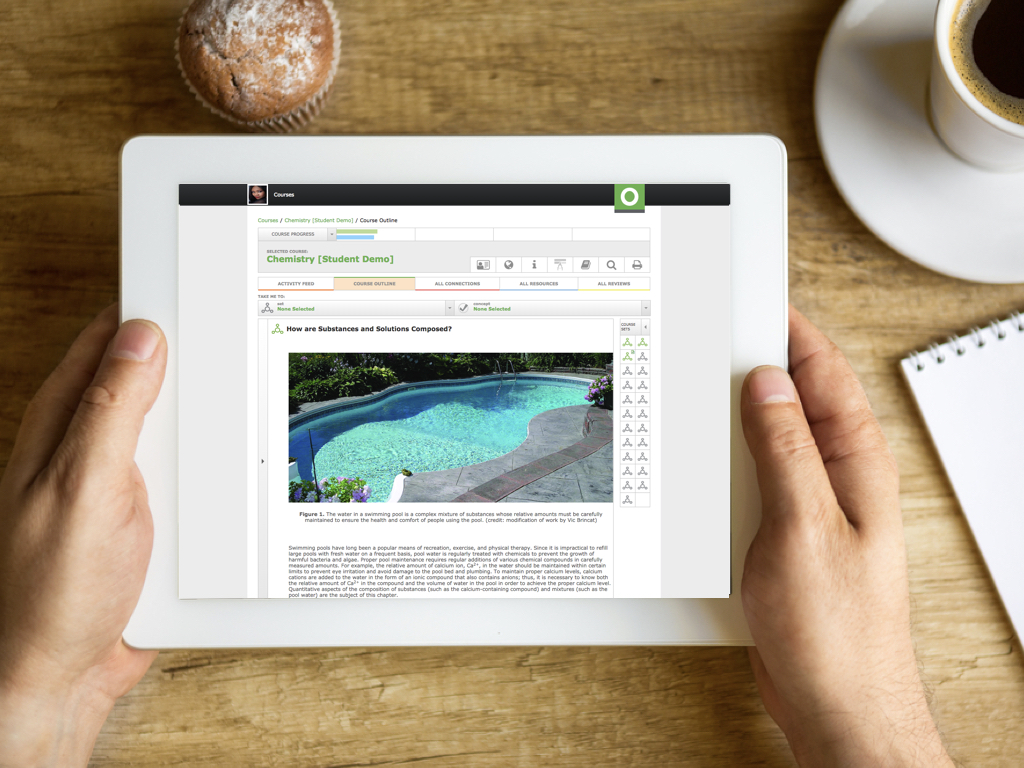
Chemistry 2e Course Outline
What are the Essential Ideas of Chemistry
- In What Contexts do we Use Chemistry?
- What are the Phases and Classifications of Matter?
- What are the Physical and Chemical Properties of Matter?
- How do we Measure Matter and Energy?
- How do we Deal with Uncertainty, Accuracy, and Precision in Measurement?
- How does Mathematics Treat the Results of Measurement?
What are Atoms, Molecules, and Ions?
- What is the History of Atomic Theory?
- How did Atomic Theory Evolve?
- How are Atoms Structured and Symbolized?
- How do we Create Chemical Formulas?
- What is the Periodic Table?
- What are Molecular and Ionic Compounds?
- What Nomenclature is Used in Naming Chemical Compounds?
How are Substances and Solutions Composed?
- How do you Calculate the Formula Mass of a Substance?
- How do you Determine the Empirical and Molecular Formulas of Unknown Compounds?
- What is Molarity?
- What are the Other Units for Solution Concentrations?
How do you Define Stoichiometry of Chemical Reactions?
- How do you Write and Balance Chemical Equations?
- How do we Classify Chemical Reactions?
- How does Stoichiometry Pertain to Chemical Reactions?
- What are the Different Types of Reaction Yields?
- In What Ways is Quantitative Chemical Analysis Performed?
How does Thermochemistry Explain the Relationship between Chemical Changes and Energy?
- What are the Basic Characteristics that Define Different Types of Energy?
- How is Calorimetry Used to Calculate and Interpret Heat?
- What is Enthalpy and How is It Calculated?
Why are the Electronic Structure and Periodic Properties of Elements Important?
- How does Electromagnetic Energy Affect Light?
- What is the Significance of the Bohr Model?
- How was Quantum Theory Developed?
- What does the Electronic Structure of Atoms Look Like?
- Why are There Periodic Variations in Element Properties?
How do Individual Atoms Connect to Form Complex Structures?
- How are Ionic Bonds Formed?
- How are Covalent Bonds Formed?
- What are Lewis Symbols and Structures?
- Why are Formal Charges and Resonance Important to Molecule Behavior?
- How are the Strengths of Ionic and Covalent Bonds Determined?
- In What Ways do Molecular Structure and Polarity Influence Molecules?
What are the Advanced Theories of Covalent Bonding?
- How is Valance Bond Theory Explained?
- What is the Association between Hybrid Atomic Orbitals and Molecular Geometry?
- How are Multiple Bonds Formed between Molecules?
- What are the Basic Approaches to Molecular Orbital Theory?
Why are the Behaviors of Gases Important to Chemistry?
- What is Pressure and How is It Measured?
- How are Pressure, Volume, Amount, and Temperature Related?
- What is the Stoichiometry of Gaseous Substances, Mixtures, and Reactions?
- Why do the processes of Effusion and Diffusion Occur?
- How is the Kinetic-Molecular Theory Explained?
- What Factors Lead to Non-Ideal Gas Behavior?
How do Molecular Interaction Affect the State of Liquids and Solids?
- What Types of Intermolecular Forces are Possible between Atoms or Molecules?
- What are the Major Properties of Liquids?
- When do Phase Transitions Occur?
- How are Phase Diagrams Constructed and Used?
- What are the Distinguishing Properties of Solids?
- How are Atoms and Ions Arranged in Lattice and Crystalline Solids?
What is the Nature of Solutions and Colloids?
- How does the Dissolution Process Occur?
- What are Electrolytes?
- How do External Factors Affect Solubility?
- What are Colligative Properties of Solutions?
- How are Colloids Formed?
How does Kinetics Influence Chemical Reactions?
- How are Chemical Reaction Rates Determined?
- What Factors Affect Reaction Rates?
- How are Rate Laws Used to Calculate Reaction Rates?
- In What Situations are Integrated Rate Laws Applied?
- How does Collision Theory Explain How Reaction Rates are Affected?
- Why are Reaction Mechanisms Important?
- What is the Function of a Catalyst?
What are the Fundamental Equilibrium Concepts?
- How is Chemical Equilibria Explained?
- What are the functions of Equilibrium Constants?
- How does Le Châtelier’s Principle Explain Shifting Equilibria?
- In What Situations are Equilibrium Calculation Used?
What are Acid-Base Equilibria?
- What are Brønsted-Lowry Acids and Bases?
- How are pH and pOH determined?
- In What Ways are the Relative Strengths of Acids and Bases Determined?
- How does the Hydrolysis of Salt Solutions Occur?
- How do Polyprotic Acids Form?
- What is the Composition and Function of Acid-Base Buffers?
- Why are Acid-Base Titrations Important?
How do the Equilibria of Other Reaction Classes Occur?
- Under What Conditions do Precipitation and Dissolution Occur?
- What are Lewis Acids and Bases?
- How can Systems Exist in Multiple Equilibria?
How Can Thermodynamics Be Used to Predict Chemical and Physical Changes?
- What Affect Does Spontaneity have on Chemical and Physical Processes?
- What is Entropy?
- What are the Second and Third Laws of Thermodynamics?
- How is Free Energy Defined and Calculated?
What Topics does Electrochemistry Cover?
- How are Oxidation-Reduction Reactions Balanced?
- What are the Basic Components of Galvanic Cells?
- How are Standard Reduction Potentials Determined?
- How is the Nernst Equation Used to Determine Cell Potentials?
- What are the Differences between Types of Batteries and Fuel Cells?
- What is Corrosion?
- What is Corrosion?
What’s the Difference Between Metals, Metalloids, and Nonmetals?
- Why is Periodicity Important?
- What Makes Up the Representative Metals?
- What Makes up Metalloids?
- What are Nonmetals Made of?
- What is Hydrogen Made of?
- What are Carbonates Made of?
- What is Nitrogen Made of?
- What is Phosphorus Made of?
- What is Oxygen Made of?
- What is Sulfur Made of?
- What are Halogens Made of?
- How can Noble Gases be Used?
How Does Transition metal Relate to Coordination Chemistry?
- What are Transition Metals Made of?
- What is the Coordination Chemistry of Transition Metals?
- How do we Find the Spectroscopic and Magnetic Properties of Coordination Compounds?
Why is Organic Chemistry Important?
- What are Hydrocarbons?
- What is Alcohol and Ethers Made of?
- What is the Property of Aldehydes, Ketones, Carboxylic Acids, and Esters?
- What is the Difference Between Amines and Amides?
What is Nuclear Chemistry?
- Why is Nuclear Structure and Stability Important?
- How Do Nuclear Equations Work?
- What is the Significance of Radioactive Decay?
- How Does Transmutation Relate to Nuclear Energy?
- How Can Radioisotopes be Used?
- What are the Biological Effects of Radiation?
Combine this content with our customizable virtual or at-home Chemistry Lab kits to create a complete digital learning experience for your students.
Chemistry 2e is designed for the two-semester general chemistry course. The textbook provides an important opportunity for students to learn the core concepts of chemistry and understand how those concepts apply to their lives and the world around them. The book also includes a number of innovative features, including interactive exercises and real-world applications, designed to enhance student learning. The second edition has been revised to incorporate clearer, more current, and more dynamic explanations, while maintaining the same organization as the first edition. Substantial improvements have been made in the figures, illustrations, and example exercises that support the text narrative.
About the authors:
Senior Contributing Authors
Paul Flowers, University of North Carolina at Pembroke
Klaus Theopold, University of Delaware
Richard Langley, Stephen F. Austin State University
William R. Robinson, PhD
Contributing Authors
Don Frantz, Wilfrid Laurier University
Paul Hooker, Westminster College
George Kaminski, Worcester Polytechnic Institute
Jennifer Look, Mercer University
Carol Martinez, Central New Mexico Community College
Andrew Eklund, Alfred University
Mark Blaser, Shasta College
Tom Sorensen, University of Wisconsin-Milwaukee
Allison Soult, University of Kentucky
Troy Milliken, Jackson State University
Vicki Moravec, Trine University
Jason Powell, Ferrum College
Emad El-Giar, University of Louisiana at Monroe
Simon Bott, University of Houston
Don Carpenetti, Craven Community College


